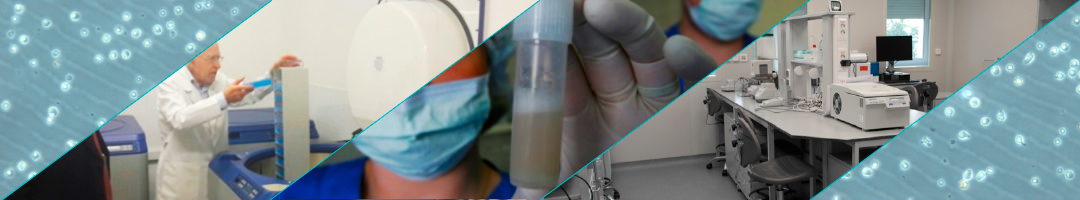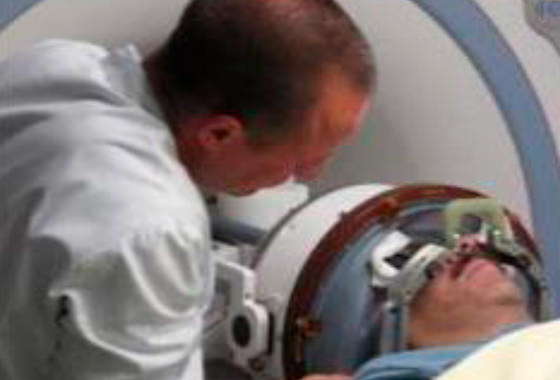
Donation and Bone
Marrow Isolation
Donation and Bone
Marrow Isolation
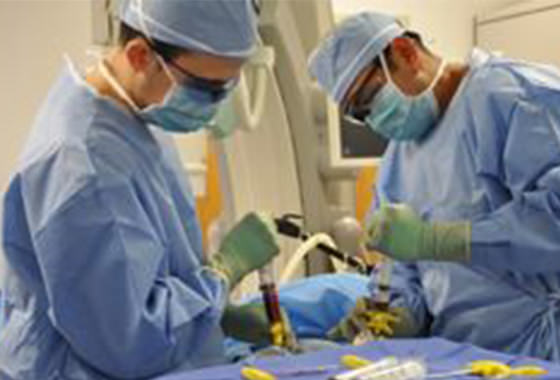
Cell Therapy
Cell Therapy
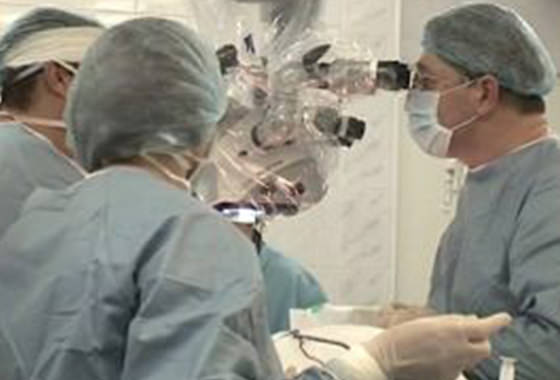
Tissue Engineering
Tissue Engineering
Patient Information
- Why are your technologies unique?
- What is regenerative medicine?
- What are stem cells and what can be treated with them?
- What types of stem cells are distinguishable and what is the difference between them?
- Can the stem cells help in the diseases and injuries of the brain and spinal cord?
- Have you confirmed the effectiveness of the stem cell therapy?
- What are the complications from stem cells? Can the tumor develop after their administration?
- How and why the stem cells are received?
- Can I donate my stem cells?
- Do you do the tissue engineering of the human brain and spinal cord?
- The review of the project Proteome-Based Personalized Anti-Cancer Cell Therapy.
-
Why are your technologies unique?
People say that if you need a surgery, you should address those hospitals where this type of surgery is put on “the assembly line” and the operation is just a daily routine for a surgeon. The experience of our doctors who administer the stem cell therapy totals 10000 patients and 15 000 infusions of the cell product. So, we know everything that can happen in the course of the cell therapy.
Our hospital is the most experienced and safe company among those who give the stem cell therapy. Our cell technologies have passed through the multiple stages of upgrading and improvement, and to date, they are the safest and most effective in the regenerative medicine. The cell product that we use is based on the hematopoietic stem cells and precursor cells that have specific molecular-biological features.
The cells can be activated with autologous exosomes (microvesicles of 30-40nm) that we isolate from the patient’s platelets according to our own technology. Activation of the hematopoietic stem cells and precursors of a patient or a healthy donor with exosomes is our new technique and the effectiveness of the cell product increases by 15%. Another unique method is the molecularly targeted biomedical cell products (MTBCP). The range of the MTBCP includes the OncoComb© MTBCP, the HemaComb© MTBCP, the NeuroComb© MTBCP. The targeted cell products affect the acceptor proteins of the signaling transduction pathways that had been modified in the course of the disease.
-
What is regenerative medicine?
According to the definition in Wikipedia the regenerative medicine is a branch of translational research in tissue engineering and molecular biology which deals with the "process of replacing, engineering or regenerating human cells, tissues or organs to restore or establish normal function", while Russian version says that it is a contemporary filed of healthcare aimed at the restoration of the structure and function of diseased or injured tissue through the activation of endogenous stem cells (SCs) or through SCs transplantation. Obviously, the latter definition is not complete and it makes the regenerative medicine deal with the cell therapy only.
It would be more correct to understand regenerative medicine as restoration, reconstruction or de novo development of the living and functioning tissue for the repair or replacement of the tissue or organ that was damaged or lost its function due to ageing or disease. The regenerative medicine is based on the use of molecular and cellular mechanisms of restoration of the structures and functions of the body and presents a fundamental basis for the medicine of future that would be able not only to substitute transplantation of the donor organs and tissues but also relieve humankind of many diseases. The range of interests of regenerative medicine is quite broad including restoration and replacement of the bone, cartilage, skin after burns, new blood vessels and even complex organs, such as liver, kidney, lungs, heart and brain in order to restore normal function.
Regenerative medicine strives after the solutions of the following issues of healthcare:
- Development of clinically meaningful protocols (GMP/GCP) for cell therapy and tissue engineering, including cell isolation, expansion and cryopreservation (biobanks);
- Development of the technologies of cell therapy (cell transplantation) and tissue engineering for regeneration of the damaged organs;
- Development of the microcellular engineering for regeneration of the organs;
- Development of the cell and protein engineering of replacing autogenetic materials and biodegradable matrixes;
- Modeling of the disease on the basis of tissue engineering;
- Development of the artificial organs and tissues for transplantation.
Regenerative medicine is an innovative field of healthcare, and it forms the complex system of the closely interrelated research, practical and social (bioethical) measures. In research and practice the regenerative medicine gives priority to novel medical devices and artificial organs, tissue engineering, cell therapy, new biomaterials and translational medicine for the quickest introduction of the progressive regenerative technologies to the clinical practice.
It seems reasonable if we say that the regenerative medicine is a clinical inter-disciplinary innovative highly-technological sphere of healthcare that relies on the generation and accumulation of specific knowledge of the ways and mechanisms of restoration and/or replacement of the damaged organs, tissues and cells, use of restorative therapeutic strategies for their reconstruction, as well as use of the diagnostic and therapeutic biotechnological platforms of tissue stimulation, cell therapy, molecular, peptide and tissue engineering, transplantation of donor and artificial organs, involving innovative critical technologies for bioreconstruction and repair of the organs and tissues damaged due to the injuries, acquired or inherited diseases (Andrey S. Bryukhovetskiy, 2017).
The object of the regenerative medicine is the personalized or differentiated and tissue-specific intra-tissular cell-by-cell restoration or replacement of the morphologic or bioinformational structure of the injured organ using novel technologies of the controllable regeneration of tissues: cell transplantation, tissue engineering, peptide and molecular bioengineering, methods of organs’ transplantation, purposeful angiogenesis, microsurgical reconstruction and other.
Clinically, the regenerative medicine includes diagnostics and complex treatment of the injuries and various diseases with conventional and transplantational methods as well as administration of the specialized cell products, cell vaccines and cell systems with specified properties. The content of the clinical discipline of regenerative medicine also includes use of surgical technologies or reconstruction of replacement of the damaged organs or their parts, or tissues with donor organs, artificial tissue engineered analogues and bioprostheses, as well as use of the extra- and intracoproreal molecular bioassembling and biodestruction of the sites of tissue in the pathological organs.
The subject of regenerative medicine is the tissue of the damaged or diseased organ of a human or animal.
The instruments of regenerative medicine are the traditional transplantation, innovative cellular, biomolecular, genomic and post-genomic (proteomic, transcriptomic, metabolomic, secretomics, RN-nomic and other) technologies, as well as micro and nanotechnologies for bioengineering repair of the analogues of the structure of the injured tissue.
-
What are stem cells and what can be treated with them?
In public opinion, the term “stem cells” is the representation of both miracle and threat. One part of public together with the leading researchers and Nobel Prize winners associates the stem cells with the anti-ageing, increased lifespan, replacement of organs and tissues and cure of the chronic disease of civilizations, including diabetes mellitus, cancer, Alzheimer’s and Parkinson’s disease, chronic injuries of spinal cord and brain, arthritis, hereditary diseases and many other. The other part along with well-known academic scientists blames the stem cells and their researchers for the onset of cancer and considers stem cells part of the conspiracy theory and global deception. Obviously, the stem cells are the most amazing discovery of the 20th century. The stem cells are simply several populations of the least specialized low-differentiated cells that provide the source of renewal for all types of tissue. These cells are distributed unevenly through the organism and can be found in all organs in small amount varying for 0.1 to 3% (the authors provide different evidence).
The specific characteristics of the stem cells:
- Self-supporting population;
- Rare division;
- Tolerance to the effects of damaging factors of the environment;
- Production of multiple progeny of several lines of specialization.
The stem cells are used by the organism gradually, under the influence of the age-related changes in the niches of the stem cells their number reduces. The number of the stem cells in some of the tissues, for example, in the stroma of bone marrow, decreases quite radically, hundredfold and thousandfold. Age-related changes in the skin, endocrine, muscle and immune systems are associated with the exhausting pool of the regional stem cells.
The extremely complicated and controversial attitude of the people to the stem cells is linked with the history of their discovery in medicine. It has been over a hundred years ago when the immigrant Russian researcher Alexander Maximov discovered a hematopoietic stem cell (HSC). However, the role of the HSCs has not been evaluated to the full that time, and the HSCs were perceived only as the cells that provide for hematopoiesis. The rediscovery of the SCs happened in the end of the 20th century when their unique abilities to repair organs and tissues have been discovered. The scientific vigor led to some kind of gold rush in the researchers and physicians, and the use of the SCs led to tragedy in some cases. The embryonic SCs have been used in the therapy of humans, they induced the development of the tumors known as teratomas and the clinical use of the SCs as well as state funding of their research have been banned by the US government. Only after 2008 the ban was lifted and the era of SCs was launched. To date the SCs is a key trend of the contemporary biotechnologies and a new clinical reality of the global medicine. The cell therapy has dramatically changed the basic therapeutic approaches to the treatment of the vascular and neurodegenerative diseases, modified the main protocols of the treatment of the injuries of the central nervous system and some of the lethal oncohematological diseases. Transplantation of the stem cells provided a theoretical and methodological platform for the development of the novel biomedical technologies in medicine.
However, the cell therapy failed to deliver on all expectations of the researchers and pioneers of the regenerative medicine who hoped for a fantastic breakthrough and opportunity to grow artificial organs in a tube.
In truth, the bioreactors for growing artificial equivalents of the skin, trachea, liver and bladder using the SCs and biopolymer matrixes have been developed but so far they are quite imperfect. However, the SCs are administered in the case of severe and almost incurable neurologic diseases (Parkinson’s, Huntington’s disease, vascular dementia, consequences of the strokes, injuries of brain and spinal cord), cardiologic diseases (miocardiodystrophy and consequences of the heart attack) and the diseases of musculoskeletal system. The HSCs are now a golden standard in the therapy of hemoblastosis and lymphoproliferative disorders, thanks to them the leucosis is cured in 95% cases in children.
-
What types of stem cells are distinguishable and what is the difference between them?
All SCs have two key characteristics: multipotency and capacity for self-renewal. The latter implies that in case of division there will be received at least one cell which is fully identical to the mother’s and retains the stem properties. In the case of symmetric division there will be received two cells of the kind, while in asymmetric one cell will have the characteristics of a stem cell, and the other will get its own program of development and begin to differentiate. The multipotency of the SCs means the capacity to generate the cells of different types. Depending on the principle of potency three groups of stem cells are distinguished: totipotent, pluripotent and multipotent (Tang et al., 2013).
The totipotent SCs can differentiate into the cells of all types and generate the viable organism. The fertilized egg also known as a zygote is a main source of these SCs. The SCs that form at the first cycles of zygote division are also totipotent and have telomerase activity. The pluripotent SCs are the direct progeny of the totipotent SCs, and they are able to differentiate almost into all types of mammalian cells and generate the germ layers of the human. The SCs of this formation are able to differentiate into the cells of different types, but only within their germ layer (Huang Н. et al., 2010).
Unipotent have not received that much attention and not all of the researchers distinguish this type of cells. The unipotent stem cells or precursor cells have the minimal potential to differentiation and are capable for multiple self-reproduction making them the long-term source of the cells of one type. Depending on their source, the practical medicine divides SCs into embryonic, fetal and post-natal or SCs of an adult organism.
We can distinguish the main types of stem cells suitable for the cell therapy of neurologic disorders. Throughout several decades various types of stem cells have already been tested and even administered to treat sever neurologic disorders. Among the first type, the human embryonic stem cells (hESCs), hematopoietic SCs, mesenchymal SCs, cell of fetal brain, umbilical cord stem cells or neural SCs derived from induced polipotent SCs are distinguished.
Embryonic stem cells (ESCs) are pluripotent SCs that from internal sell mass at early stage of embryo development. Apart from their high potential to differentiation, other important feature is absence of human leukocyte antigen (HLA) expression. This helps avoid rejection by the immunocompetent organism of the recipient. However, isolation of ESCs entails elimination of embryos which induces serious ethics and religious debates and limitations leading to legal issues. Possible solution is offered by the method of reprogramming of somatic cells based on the nucleus transfer of the somatic cell into oocyte cytoplasm. However, there is no clear answer to the question about clinical use of the cell systems of this type. The hESCs have the opportunities to differentiate in any type of the cells, they are polipotent. Since 1998, when the first line of hESCs was established, they are widely used as an unlimited source of the cells for the research in the sphere of evolutionary biology, drug development, transplantation and regenerative medicine. Before their full potential for the cell replacement therapy can be realized, they have to differentiate into specialized progeny cells, and their safety and efficiency requires accurate research, so that such barriers as heritable resistance, teratoma and functional disintegration could be avoided.
The source of the fetal SCs as it follows from their name is the fetus received in the abortion at 9 to 12 weeks of gestation. Apart from ethics complexities, the use of this material is associated
with the risk of infecting the patient (Stupp et al., 2013). In Russia use of the ESC and fetal SCs is banned since 2003.
Postnatal SCs are the main material for regenerative medicine. The opportunity to isolate them directly from the patient helps avoid ethic and legal discussions while from the medical point of view the autogenetic material does not induce the graft rejection. Postnatal SCs can be subdivided into three groups: hematopoietic SCs (HSCs), mulipotent mesenchymal stromal cells (MMSCs) of bone marrow and tissue specific progenitor cells (Kim et al., 2012). The HSCs are multipotent cells that give rise to all cells of blood. The main source of the HSCs is bone marrow that contains three subpopulations of the HSCs: lymphoid-oriented, myeloid-oriented and balanced. The HSCs can be obtained directly from peripheral blood after provisional processing with cytokines, including granulocyte colony-stimulating factor that induces exit of HSCs into peripheral blood.
The HSCs have been successfully used in medicine for over 60 years. Originally, they have been used in oncology to suppress purulent-septic complications and to remodel hematopoietic system after high-dose chemotherapy. Later it has been proposed to model transplant versus tumor reaction with HSCs. To date the HSCs are the main tool of regenerative medicine. Their ability to migrate to the site of injury allows stepping away from traditional methods of transplantation to the site of injury where the intercellular relations are violated and patient’s own cell elements are dead or damaged by pathological process. Targeted migration of HSCs to the site of injury lets HSCs interact with the damaged cells, and this results in the launch of the program of survival, regeneration or apoptosis.
-
Can the stem cells help in the diseases and injuries of the brain and spinal cord?
For a long time it has been assumed that regeneration and restoration of the brain and spinal cord is impossible because nervous cells cannot restore. The stand point of contemporary neuroscience that regeneration of the brain and spinal cord in mammals is absent and is not possible, traces its roots to the fundamental research of the Nobel Prize winner in medicine and physiology Santiago Ramón y Cajal. The Spanish physician and histologist studied neurogenesis in the end of the 19th century and developed his theory of neural organization of the brain and proved that neural cells do not regenerate.
Since then and to the very end of the 20th century these postulates about neurogenesis and regeneration in the central nervous system have been domineering in neurology, neurosurgery and psychiatry. All generations of neurologists, neurosurgeons and neuroscientists knew this fact as self-evident: regeneration of the brain and spinal cord is not possible, as it is not possible in all stationary tissues, and primarily in the CNS.
In early 21st century it has been proved that regeneration of the brain or spinal cord in adults is feasible and neural cells are able to regenerate under the effect of the SCs that arrive to the site of brain or spinal injury.
The ability of the SCs to forward to the site of the ischemic or neoplastic damage in the brain has been first demonstrated by the teams of Sarah Benedetti and Karen Aboody. Seventy nine chemical compounds (cytokines and chemoattractants) have been identified as well as over 20 types of molecultrar-biological receptors that control processes of the targeted migration of various SCs in health and in disease have been described (Franco et al., 2013). The central role is played by the interaction of the stromal cell-derived factor (SDF-1α) with CXCR4 receptor. The role of various ligand receptor interactions of stem cell factor (SCF) with c-Kit receptor is being discussed, as well as hepatocyte growth factor (HGF) with c-Met receptor, Vascular endothelial growth factor (VEGF) with VEGFR receptor, monocyte chemoattractant protein 1 (MCP1) with CCR2 receptor, High mobility group box 1 protein (HMGB1) with RAGE receptor and many other protein-protein-interactions.
Damage of the brain or spinal matter induces inflammatory response represented as recruiting of neutrophils, monocytes and macrophages to the site of injury, and expression of a multiple chemokines, cytokines and metalloproteases. When HMGB1 is released from the site of injury, it interacts with RAGE receptor of the stem cells surface and enhances the production of the tumor necrosis factor (TNF), interleukins IL1 and IL8, MCP-1, SDF-1α and other factors that recruit healthy SCs.
So, the research of the molecular-biological mechanisms of migration and targeted navigation of the SCs to the site of injury of various etiology and pathogenesis offer a new stand point on the processes of regeneration and sanogenesis in the brain. The pronounced migration of the SCs to the site of ischemic, traumatic and neoplastic damage is a multi-level regulatory mechanism to support the tissue balance. This makes stem cells leave their niches and migrate to the site of injury where they modulate the processes of program cell death, proliferation and differentiation.
These are the mechanisms of SCs action that promote restoration of the damaged neurons:
- The SCs merge with the damaged cell and all its genetic material is used to restore the damaged neural cells;
- The SCs regulate functional state of a neuron, expressed biologically active substances, improve trophic function of the damaged cells and trigger processes of self-reparation and restoration.
- The SCs replace damaged neural cells, differentiate appropriately and take the place of damaged cells.
-
Have you confirmed the effectiveness of the stem cell therapy?
We have been using stem cells to treat nervous and oncologic diseases for 25 years and for 16 years we administer the hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) to treat the traumatic disease of the brain and spinal cord. Our evaluation of effectiveness of the stem cells therapy varies from 42 to 65%. The cell therapy in mine-blast trauma and combat trauma was effective in 42 to 52% in 350 cases. The effectiveness of the cell therapy in severe traumatic injuries of brain and spinal cord is about 60% over 3500 cases of civilian population.
The outcome of the therapy of organic injuries of the brain and spinal cord is significantly influenced by timely primary surgical debridement, degree of restoration of the anatomy of the spinal column and skull, as well as the degree of restoration of the cerebrospinal fluid flow in the central nervous system. The effectiveness of the SC therapy increases with the specialized rehabilitation and when we add exosomes that have been isolated from the platelets of the patient.
-
What are the complications from stem cells? Can the tumor develop after their administration?
There is no medicine without complications and even common intramuscular injection can lead to tragedy. So it would be a lie if we say that there are no complications from cell therapy. Mostly, the complications develop when the technology of preparing the product in the laboratory is violated or the cell product is administered in an incorrect way. Also, the complication may arise, when the patient does not follow doctor’s sanitary or regimen recommendations. All complications of the spinal tap such as headaches, dizziness, CSF leakage, meningeal events of irritated membranes and other can be easily coped with under the in-patient conditions.
The stem cell induced tumor can develop only in the case of administration of the embryonic SCs isolated from the human embryo blastocyte, and their use is banned in Russia. In theory, the tumor can develop from adult SCs if the cells will have four and more specific mutations induced by gene engineering, ionizing radiation, carcinogens processing, viral or bacterial contamination; in this case they turn into the cancer stem cells.
However, we have shown that three adult HSCs and HPCs can block the stem call of such cancers as breast, lung cancer and glioblastoma multiforme and that is what we based our therapy on. In the therapy we use the concentrate of the mobilized mononuclear cells of peripheral blood that are the main killer cells of the inherent immunity (17 to 20 % of NK and NKT cells and 10 % γδ Т cells) that significantly enhances the anti-cancer characteristics of the cell product.
-
How are the stem cells isolated and why?
For the past 16 years our team has been using the hematopoietic stem cells (HSCs) and hematopoietic precursor cells (HPCs) that are isolated either directly from the bone marrow or in the standard procedure of leukocytapheresis from the peripheral blood. Bone marrow isolation is a serious surgical procedure that requires general anesthesia or the spinal anesthesia. The bone marrow is isolated from the iliac crest with special needles, then processed and cryopreserved.
The other method is much simpler and is better tolerated by the patients. The leukocytapheresis is a well known and widely used procedure based on the separation and isolation of the lymphocytes from the blood and return of other blood components to the host. In the procedure the blood is being taken from the vein by the dual-lumen catheter and forwarded to the special equipment for leukocytapheresis. The necessary components are separated and the blood is returned back to the same vein. The separated components of blood are cleared from other remaining components in rather complex laboratory procedures and cryopreserved.
-
Can I donate the stem cells?
Usually, the patients donate the stem cells themselves in the case of the therapy of neural diseases with the hematopoietic stem cells (HSCs). However, in some cases, like in the treatment of the amyotrophic lateral sclerosis, we administer only the cells of close relative and we have our reasons for it.
Also in the treatment of malignant disorders we administer the patient’s own cell, also named autologous cells, but we temporarily change the gene expression in them by processing with perturbagens (low molecular compounds) or exosomes with perturbagens. We also use the donor cells that are matched in a molecular-biological way by our laboratory experts. So, if you want to, you can donate the cells in our hospital.
-
Do you perform the tissue engineering of brain and spinal cord?
We performed 52 successful surgeries in tissue engineering in eight years. All of them had no complications and were effective in the restoration of the spinal cord functions in 57%. The surgeries involved implantation of the US and Russia patented neuroendoprosthetic system that consists of the approved biopolymer matrix Spherogel©, hematopoietic precursor cells, neural stem cells and/or glioolfactory cells of the nasal mucosa.
-
The review on the Proteome-Based Personalized Anti-cancer Cell Therapy
The development of the proteome-based personalized cell therapy against cancer is extremely important for contemporary oncology. For the past hundred years the neurooncologists have been developing the technologies of effective elimination of the tumor cells in brain malignancies in any possible way but to no avail. The example of malignant glial tumors and metastases to the brain clearly demonstrates the insufficient effectiveness of the conventional strategy that includes neurosurgery, radio and chemotherapy. The survival median in the glioblastoma remains unchanged in the past hundred years and varies from 12 to 15 months, while in the case of metastases to the brain the survival varies from 6 to 8 months.
The key idea of the proposed technology lies in the radical change of the paradigm of treatment that has always been aimed at the complete elimination of all cancer cells. According to Prof. Bryukhovetskiy, this paradigm dominates oncology for centuries must be replaced with a new one that aims at the transfer of an acute malignant and lethal disease into a chronic and non-lethal by means of the control and regular monitoring of the number of the tumor cells using the well-known methods (neurosurgery, chemo- and radiotherapy and immunotherapy) along with the novel personalized therapy by autologous stem cells with modified proteome.
The development of this technology became possible thanks to the broad expertise of the research team that involved the application of the adult stem cells in neurooncology, as well as development of the proteome-based personalized anti-cancer cell products (PBPACP) with the specifically modified properties to be used in the complex therapy of glial tumors of the brain and metastases to the brain of breast and lung cancer. For seven years the researchers have studied cellular and molecular mechanisms of migration, homing, cell adhesion and pathotropism of adult stem cells to cancer cells in vitro and in vivo. They performed comparative proteome mapping of the proteins (PMP) and whole transcriptome profiling of the gene expression (WTPGE) of neural stem cells (CD133+) that have been isolated from the olfactory sheath of a nose, mobilized autologous hematopoietic stem cells (CD34+) and cancer stem cells (CSCs) (CD133+) isolated from the glial tumors of human brain (U87 and U251 lines) and rodent brain (C6), as well as the bioinformational processing and mathematical modeling of the results. The results of the research highlighted the global structural proteomic and transcriptomic differences between the cells. New evidence have been found that permitted development of a novel platform of the personalized regulatory influence of the specifically set secretomes of autologous SCs with modified WTPGE on the reproductive and proliferative functions of the CSC.
The research showed that the patient’s SCs always arrive to the brain tumor, find the cancer cells and cancer stem cells, adhere to them and affect according to the by-stander effect.
- Low effectiveness of the anti-cancer regulatory and controlling influence of the SCs on the CCs and CSCs is conditioned by the systemic mechanisms of speciation in carcinogenesis that can be overcome by the specified modification of effector properties of the SCs based on the results of the PMP and WTPGE.
- It has been shown that certain number of specific proteins varying from 30% to 60% can be mapped in the CSC proteome, and their bioinformational analysis allows for detection of intracellular pathways of signaling transduction (ICPST) in the CSCs that have been left unaffected by the carcinogenesis and remained accessible for targeted regulatory effect.
- Control and management over proliferative and reproductive functions of the CSCs can be achieved through the influence on the known membrane proteins of these ICPST in the CSCs.
- Available databases of the protein-protein interactions help define the main protein ligands that are able to activate the ICPST of target membrane proteins and manage the effector functions of the CSCs.
- Chemical induction allows for modification of the transcriptomic profiles of the patients SCs in the specific purposes avoiding gene engineering, so that the cell product of autologous stem cells secretes the prerequisite regulatory protein ligands.
- Special software has been developed to search the Affymetrix (UK) transcriptome database of protein-protein interactions for perturbagens that are able to appropriately modify the WTPGE of the stem cells of the patients.
The effectiveness of the proposed therapy with WTPGE modified cell systems in the suppression of the reproductive and proliferative properties of the cancer cells and cancer stem cells in the glial tumors of the brain has been demonstrated in the experiments with brain tumor models. The role and place of the proposed cytoregulatory method of anticancer treatment in the complex conventional therapy that is followed by the immune therapy of the brain tumor have been shown. Currently, the technology is patented in the Russian Federation (Patent №2535972 dated 2014), the approvals of the Scientific Boards and Ethics Committees of the Russian Academy of Science and Federal Medical-Biological Agency of Russia have been received. The project is registered at the www.clinicaltrail.gov ( NCT01782287 for Proteome-based Immunotherapy of Lung Cancer Brain Metastaseshttp://clinicaltrials.gov/ct2/show/NCT01782287?tenn=NCT01782287&rank=l.NCT017 82274 for Proteome - based Immunotherapy of Brain Metastases from Breast Cancer http://clinicaltrials.gov/ct2/results7term =NCT01782274+&Search=Search NCTO1759810 for Proteome-based Personalized Immunotherapy of Glioblastoma,
.Professor Mikhail Ivanovich Davydov is a surgical oncologist, Soviet and Russian Scientist, retired Director of the Blokhin Russian Cancer Research Center, member of the Russian Academy of Science, ex-President of the Russian Academy of Science
Patients’ admission area
-
 Reception of the Rehabilitation Department
Reception of the Rehabilitation Department -
 General view of the Neurology Department
General view of the Neurology Department -
 Enhanced comfort ward
Enhanced comfort ward -
 Intensive Care Unit of the Resuscitation Department
Intensive Care Unit of the Resuscitation Department -
 The lobby of the Neurology Department
The lobby of the Neurology Department -
 Intensive Care Unit of the Neuroresuscitation Department
Intensive Care Unit of the Neuroresuscitation Department
-
 Hall of the Department of Immunology
Hall of the Department of Immunology -
 Rehabilitation gym
Rehabilitation gym -
 The lobby
The lobby -
 Intensive Care Unit
Intensive Care Unit -
 The gym
The gym -
 The lobby of the Immunology Department
The lobby of the Immunology Department
Equipment for Cell Therapy
-

The Bank of the
Hematopoietic Stem Cells. -

The Bank of the
Hematopoietic Stem Cells. -

The Bank of the
Hematopoietic Stem Cells. -

The Bank of the
Hematopoietic Stem Cells. -

The Bank of the
Hematopoietic Stem Cells. -

The Bank of the
Hematopoietic Stem Cells.
-

The liquid nitrogen container to store the cell lines
-

The liquid nitrogen container to store the cell lines
-

Programmed cell freezer
-

Controlled Rate Freezer.
-

Liquid Nitrogen Storage
-

Cell Culture Laboratory
Dear ladies and gentlemen!
-

We guarantee the safety of the therapy with our cell products that contain the unmanipulated hematopoietic stem and precursor cells with potent anti-tumor characteristics and outstanding abilities to regenerate the damaged organs and tissues. The cultured cell products are used only for cancer and other malignancies, as the potential risks are reduced by the disease itself.
We hope that the content of our webpage prove helpful to you!
Sincerely yours,
Professor Andrey S. Bryukhovetskiy -

The review on the Proteome-Based Personalized Anti-cancer Cell Therapy
The development of the proteome-based personalized cell therapy against cancer is extremely important for contemporary oncology. For the past hundred years the neurooncologists have been developing the technologies of effective elimination of the tumor cells in brain malignancies in any possible way but to no avail. The example of malignant glial tumors and metastases to the brain clearly demonstrates the insufficient effectiveness of the conventional strategy that includes neurosurgery, radio and chemotherapy. The survival median in the glioblastoma remains unchanged in the past hundred years and varies from 12 to 15 months, while in the case of metastases to the brain the survival varies from 6 to 8 months.
The key idea of the proposed technology lies in the radical change of the paradigm of treatment that has always been aimed at the complete elimination of all cancer cells. According to Prof. Bryukhovetskiy, this paradigm dominates oncology for centuries must be replaced with a new one that aims at the transfer of an acute malignant and lethal disease into a chronic and non-lethal by means of the control and regular monitoring of the number of the tumor cells using the well-known methods (neurosurgery, chemo- and radiotherapy and immunotherapy) along with the novel personalized therapy by autologous stem cells with modified proteome.
The development of this technology became possible thanks to the broad expertise of the research team that involved the application of the adult stem cells in neurooncology, as well as development of the proteome-based personalized anti-cancer cell products (PBPACP) with the specifically modified properties to be used in the complex therapy of glial tumors of the brain and metastases to the brain of breast and lung cancer. For seven years the researchers have studied cellular and molecular mechanisms of migration, homing, cell adhesion and pathotropism of adult stem cells to cancer cells in vitro and in vivo. They performed comparative proteome mapping of the proteins (PMP) and whole transcriptome profiling of the gene expression (WTPGE) of neural stem cells (CD133+) that have been isolated from the olfactory sheath of a nose, mobilized autologous hematopoietic stem cells (CD34+) and cancer stem cells (CSCs) (CD133+) isolated from the glial tumors of human brain (U87 and U251 lines) and rodent brain (C6), as well as the bioinformational processing and mathematical modeling of the results. The results of the research highlighted the global structural proteomic and transcriptomic differences between the cells. New evidence have been found that permitted development of a novel platform of the personalized regulatory influence of the specifically set secretomes of autologous SCs with modified WTPGE on the reproductive and proliferative functions of the CSC.
The research showed that the patient’s SCs always arrive to the brain tumor, find the cancer cells and cancer stem cells, adhere to them and affect according to the by-stander effect.
- Low effectiveness of the anti-cancer regulatory and controlling influence of the SCs on the CCs and CSCs is conditioned by the systemic mechanisms of speciation in carcinogenesis that can be overcome by the specified modification of effector properties of the SCs based on the results of the PMP and WTPGE.
- It has been shown that certain number of specific proteins varying from 30% to 60% can be mapped in the CSC proteome, and their bioinformational analysis allows for detection of intracellular pathways of signaling transduction (ICPST) in the CSCs that have been left unaffected by the carcinogenesis and remained accessible for targeted regulatory effect.
- Control and management over proliferative and reproductive functions of the CSCs can be achieved through the influence on the known membrane proteins of these ICPST in the CSCs.
- Available databases of the protein-protein interactions help define the main protein ligands that are able to activate the ICPST of target membrane proteins and manage the effector functions of the CSCs.
- Chemical induction allows for modification of the transcriptomic profiles of the patients SCs in the specific purposes avoiding gene engineering, so that the cell product of autologous stem cells secretes the prerequisite regulatory protein ligands.
- Special software has been developed to search the Affymetrix (UK) transcriptome database of protein-protein interactions for perturbagens that are able to appropriately modify the WTPGE of the stem cells of the patients.
The effectiveness of the proposed therapy with WTPGE modified cell systems in the suppression of the reproductive and proliferative properties of the cancer cells and cancer stem cells in the glial tumors of the brain has been demonstrated in the experiments with brain tumor models. The role and place of the proposed cytoregulatory method of anticancer treatment in the complex conventional therapy that is followed by the immune therapy of the brain tumor have been shown. Currently, the technology is patented in the Russian Federation (Patent №2535972 dated 2014), the approvals of the Scientific Boards and Ethics Committees of the Russian Academy of Science and Federal Medical-Biological Agency of Russia have been received. The project is registered at the www.clinicaltrail.gov ( NCT01782287 for Proteome-based Immunotherapy of Lung Cancer Brain Metastaseshttp://clinicaltrials.gov/ct2/show/NCT01782287?tenn=NCT01782287&rank=l.NCT017 82274 for Proteome - based Immunotherapy of Brain Metastases from Breast Cancer http://clinicaltrials.gov/ct2/results7term =NCT01782274+&Search=Search NCTO1759810 for Proteome-based Personalized Immunotherapy of Glioblastoma,
.Professor Mikhail Ivanovich Davydov is a surgical oncologist, Soviet and Russian Scientist, retired Director of the Blokhin Russian Cancer Research Center, member of the Russian Academy of Science, ex-President of the Russian Academy of Science
Licenses, Patents and Certificates
Research Center of Stem Cells and Regenerative Medicine

Russia, Moscow, Kashirskoye Highway 23